The research team led by Prof. LIU Zhongmin, WEI Yingxu and XU Shutao from National Engineering Laboratory for Methanol to Olefins, Dalian National Laboratory for Clean Energy,Dalian Institute of Chemical Physics of the Chinese Academy of Sciences,together with Prof. ZHENG Anmin group from Innovation Academy forPrecision Measurement Science and Technology, Chinese Academy of Sciences,has recently made a new progress in the reaction mechanism of the first C-C bond formation during the Methanol-to-Olefins (MTO) process. This new finding was publishedas a research articleonline inChem.(doi:10.1016/j.chempr.2021.05.023).
As an important coal chemical process, MTO is an important reaction in C1 chemistry. The formation of the first C-C bond from methanol or dimethyl ether of C1 species in MTO reaction has always been a challenging and controversial issue in C1 chemistry.Since the formationof first C-C bond takes place at the very initial stage of the reaction, it is difficult to capture intermediate species, and there is no direct evidence for the reaction mechanism proposal. In the previous work, the methyleneoxy-analogue species,originated from activateddimethyl ether(DME),was successfully observeddirectlybyin situssNMR spectroscopy on ZSM-5 molecular sieves with MFI topological structureand recognized to be the most crucial step for the direct coupling of C1 reactants and intermediates.(Angew. Chem. Int. Ed. 2017, 56, 9039-9043)

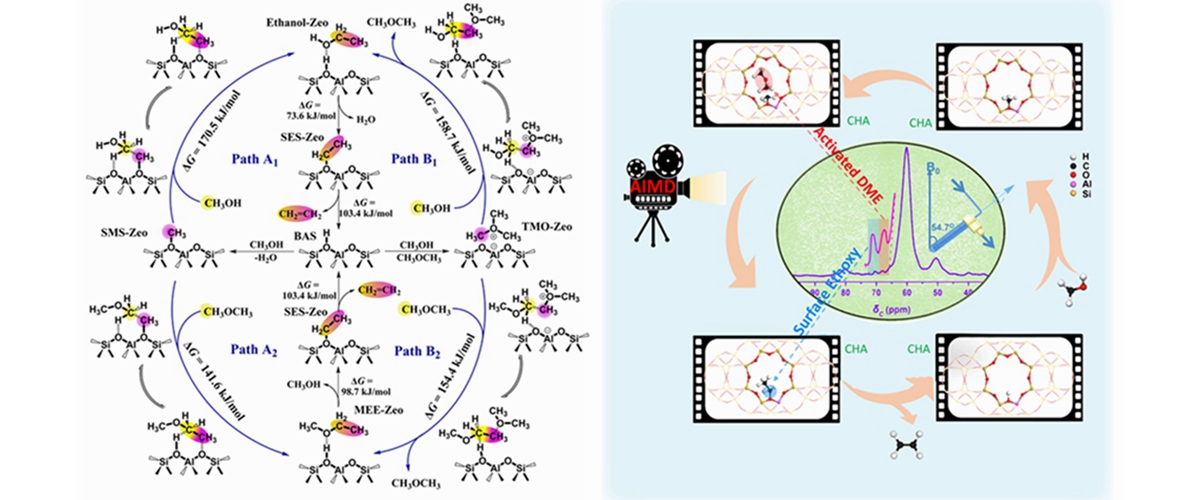
In this work, the direct C-C bond formation mechanism during initial MTO reaction over SSZ-13 zeolite with 8-membered ring and CHA topological structure was studied. The evolution of the organic species on SSZ-13 catalyst surface during the methanol conversion were detected byex situandin situsolid-state NMR techniques. For the first time, the surface ethoxy specie (SES), the critical species containing initial C-C bond, wasdirectly captured under real MTO reaction conditions at very initial reaction stage. Moreover, based on the observation and capture of C1 reactants (methanol and DME), C1 reaction intermediates (methoxy andtrimethoxyonium ion), C1 species in their activated state (methyleneoxy analogues) and initial olefin precursors (SES) on the catalyst surface, the advanced ab initio molecular dynamics (AIMD) theoretical calculation technology was employed to predict and presented thevisualizedand complete process of the initial C-C bond formation starting from C1 reactants (methanol and DME) and C1 intermediates (SMS and TMO). Based on the experimental and theoretical evidences, complete and feasible initial C-C bond formation routes, namely, SMS/TMO-mediated methanol/DME activation with the synergic effect from SMS and negatively charged framework oxygenatoms, were established.Thesenew findingnot only shed light on the controversial issue of the first C-C bond formation in MTH reaction but also enriched the fundamental theory of C1 catalytic chemistry.
This work was supported by National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Sciences, iChEM, the Youth Innovation Promotion Association of the Chinese Academy of Sciences and Liaoning Revitalization Talents Program.(Text and picture by XU Shutao& WEI Yingxu)
The first carbon-carbon bond formation mechanism in methanol-to-hydrocarbons process over chabazite zeolite, TantanSun,WeiChen, ShutaoXu, AnminZheng*, XinqiangWu, ShuZeng, NanWang, XiangjuMeng, YingxuWei*, ZhongminLiu*.10.1016/j.chempr.2021.05.023.