Aromatics are important bulk chemicals that are primarily produced from petroleum via catalytic reforming or cracking.Develpoment of a new route for aromatics synthesis from non-petroleum resources, such as coal, natural gas or biomass, is very important due to the increase in market demands for aromatics and the depletion of petroleum resources.Syngas (a mixture of CO and H2), derived from coal, natural gas, or biomass, is utilized as a platform for aromaticssynthesis. Recently, a bifunctional compositecatalyst of metal oxide and zeoliteexhibited excellent aromatic selectivity in the syngas to aromatics (STA) reaction, but the mechanism on the formation of aromatics from syngas remains controversial.
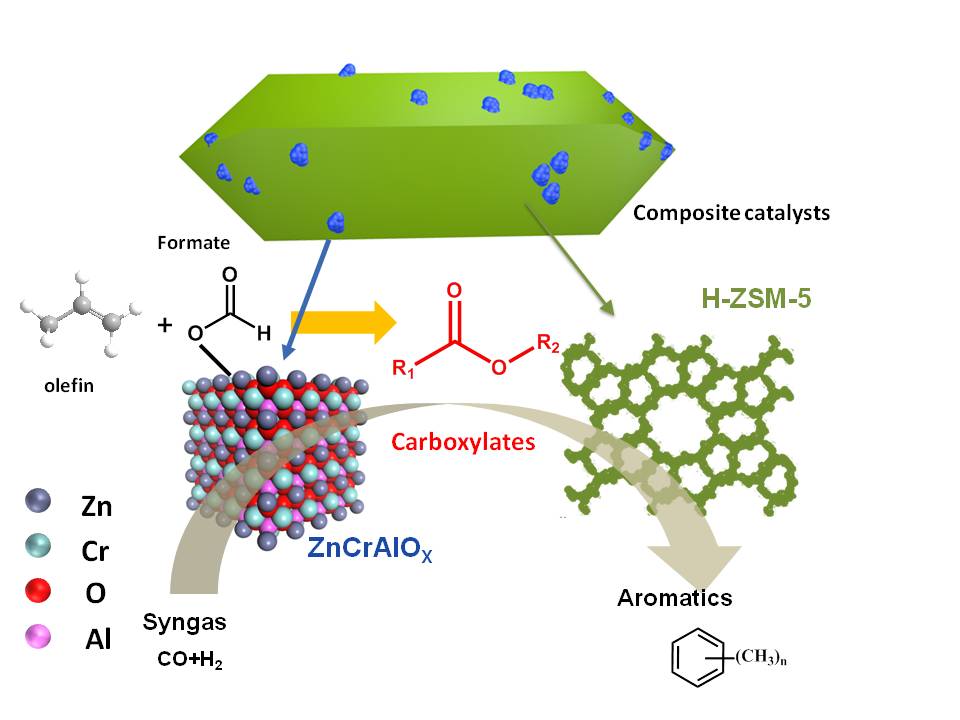
Recently, a research group led by Prof. Liu Zhongmin from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences revealed the aromatics formation mechanism from syngas over bifunctional catalysts.In this work, it was reported that the carboxylates, formed by the reaction between formate species and olefins, promote the aromatizationprecess in syngas conversion reactions over a ZnCrAlOx&H-ZSM-5 composite catalyst. In addition, it is shown that the carboxylates favor the formation of aromatics over H-ZSM-5 even in the presence of H2. A novel mechanism for the formation of aromatics via the generation and transformation of carboxylate intermediates is proposed, and the transformation of carboxylates to aromatics via methyl-2-cyclopenten-1-one (MCPO) intermediates is indeed likely. A better understanding of the formation mechanism of aromatics would help optimize the composite catalyst.
The study was published in Chinese Journal of Catalysis on May 2020.
The carboxylates formed on oxides promoting the aromatization in syngas conversion over composite catalysts. Zhiyang Chen, Youming Ni, Fuli Wen, Ziqiao Zhou, Wenliang Zhu*, Zhongmin Liu, Chinese Journal of Catalysis, 42, 2021.